Introduction: Noninvasive prenatal testing (NIPT) is routinely used to screen for fetal aneuploidies in chromosomes 13, 18 and 21. NIPT uses NGS based methods to detect fetal cfDNA in maternal circulation. In 2016, the American College of Obstetricians and Gynecologists recommended offering NIPT testing for all pregnant patients regardless of maternal age. Reports of maternal malignancy detected by NIPT have been increasing since its wide adoption into clinical practice, but subsequent management has not been standardized. We therefore report the outcomes of patients with abnormal NIPT evaluated and managed at a single institution.
Methods: A case series ofpatients referred to Dana-Farber Cancer Institute between 2014 and 2022 for evaluation following an abnormal NIPT or for treatment of a cancer identified through NIPT.
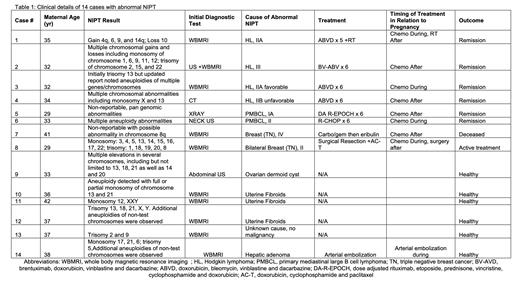
Results: Fourteen cases were included in this series. The median age at NIPT was 34 (range 29-42). Most patients (79%) had multiple chromosomal aneuploidy while 3 patients had ≤2 chromosomal abnormalities reported on their NIPT. Twelve patients (86%) underwent subsequent invasive prenatal testing: results were normal in all but one whose trisomy 12 finding led to early termination. Eleven patients (79%) underwent a whole-body MRI (WBMRI), 6 (55%) of which identified a suspicious finding that required biopsy. Of the 6 patients biopsied for a finding on WBMRI, 5 (83%) were found to have a malignancy. All patients who had a negative WBMRI did not have an identified malignancy. Of the total 14 cases, 10 (71%) underwent biopsies for abnormal findings and 8 (80%) of biopsies led to diagnosis of malignancy (or 57% of overall cohort). This was found to be Hodgkin lymphoma in 4 (29%), primary mediastinal large B cell lymphoma in 2 (14%) and triple negative breast cancer in 2 (14%) of the patients. Other causes of abnormal NIPT were uterine fibroids in 3 (21%), hepatic adenoma in 1 (7%), ovarian dermoid cyst in 1 (7%) and no abnormal findings in 1 (7%). Half of the patients received cancer treatment during pregnancy and the other half after pregnancy. Treatment included chemotherapy 6 (75%), chemotherapy/radiation 1 (12.5%) and surgery/chemotherapy 1 (12.5%). Post treatment, 6/8 (75%) patients achieved a complete metabolic response (CMR), one patient is still undergoing treatment and one patient (breast cancer) is deceased. Overall, there were 12 (86%) live births, one pregnancy termination and one patient with missing data. The median birthweight was 7.2 lbs (6.1-8.5 lbs). Two patients had NIPT with previous pregnancies. One patient had a normal NIPT and the other had an abnormal NIPT that was similar in two pregnancies, but she had a hepatic adenoma. Two patients with PMBCL had subsequent pregnancies and repeat NIPT after treatment were both normal .
Conclusions: Specific abnormal NIPT results may be caused by maternal malignancy, especially where there is discordance with the fetal karyotype. A WBMRI without contrast is a safe and feasible initial approach for diagnosis and staging in these patients. Most tumors are lymphomas, for which treatment during and soon after pregnancy is possible with favorable maternal and fetal outcomes.
Disclosures
LaCasce:Research to Practice: Consultancy; Seagen, Kite Pharma: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal